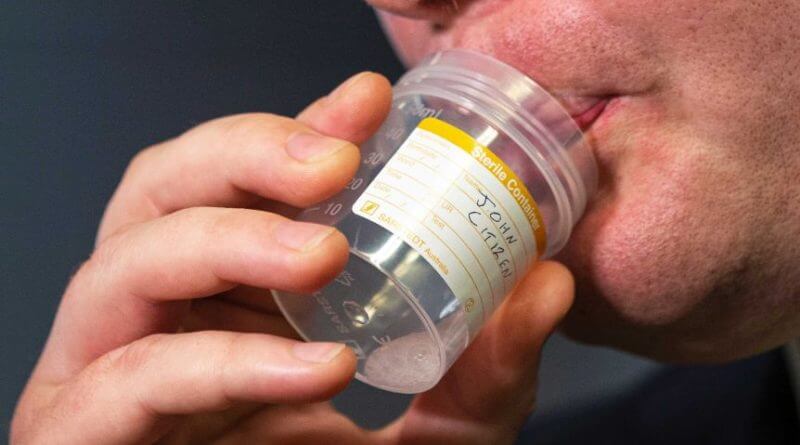
FDA Grants Emergency Use of Saliva-Based COVID-19 Test
On Saturday, the Food and Drug Administration approved emergency use to a new COVID-19 test that is based off of saliva samples.
This new test is hailed as being quicker and inexpensive compared to the others currently in use by medical centers.
SalivaDirect, which is the name of the new test, has yielded similar results compared to the nasal swab in trials. Patients in this test simply give a saliva sample in a sterile container under the supervision of medical personnel.
The test can deliver results in up to three hours, and about 92 samples can be tested at a single time. The NBA has been instrumental in funding and delivering this test.
White House COVID-19 Testing Coordinator Dr. Brett Giroir issued a statement.
“The SalivaDirect test for rapid detection of SARS-CoV-2 is yet another testing innovation game changer that will reduce the demand for scarce testing resources.”